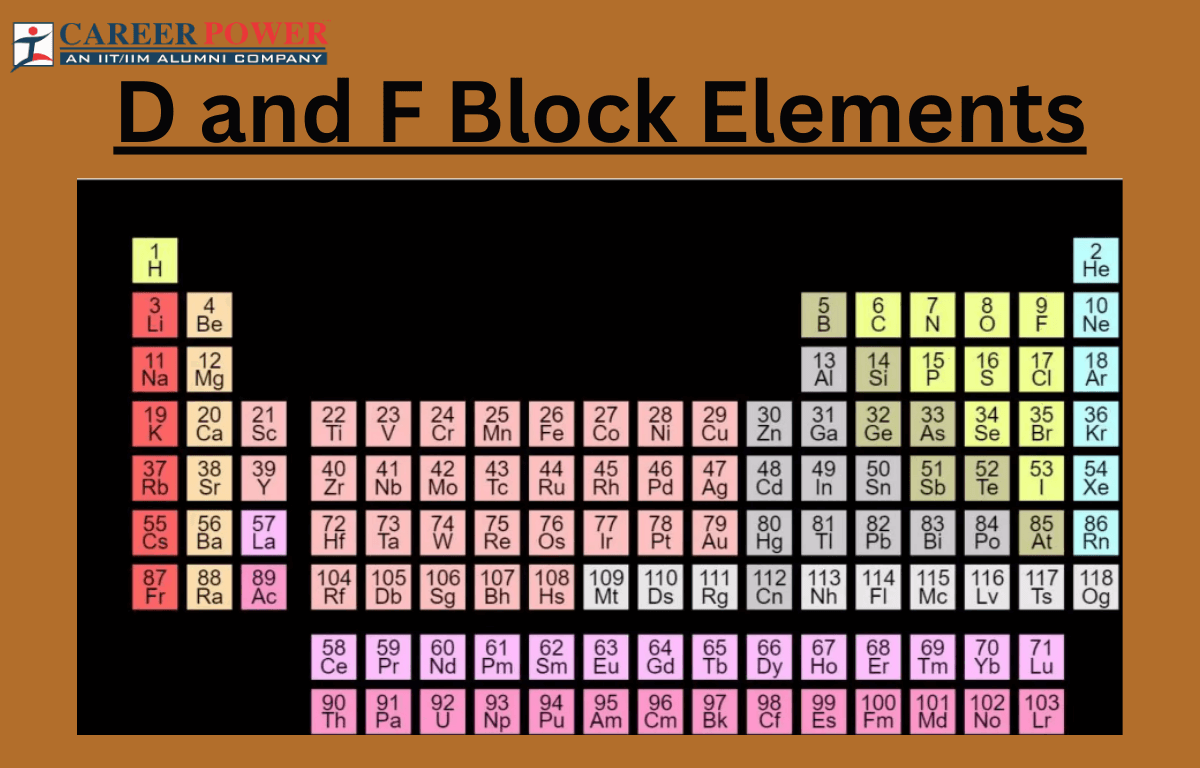
D-block: transition metals, versatile and form colorful compounds. F-block: lanthanides and actinides, inner transition metals with unique properties and applications. More details are mentioned here.
Published On January 31st, 2024

Table of Contents
The d and f blocks, often referred to as transition metals, respectively, are two crucial sections in the periodic table. The d block elements encompass elements with partially filled d orbital, typically filling up in the penultimate electron shell. Transition metals in this block exhibit diverse oxidation states, form colorful compounds, and often serve as catalysts due to their electron configuration. On the other hand, the f block consists of lanthanides and actinides, which occupy the two rows at the bottom of the periodic table. The orbitals have f orbital gradually filling up, leading to unique magnetic properties and characteristic electron configurations.
Both d and f block elements play pivotal roles in various chemical reactions, industrial processes, and technological applications, making them essential components of the periodic table. Their versatility stems from the availability of multiple oxidation states and the ability to form complex compounds, contributing significantly to the richness and complexity of chemistry.
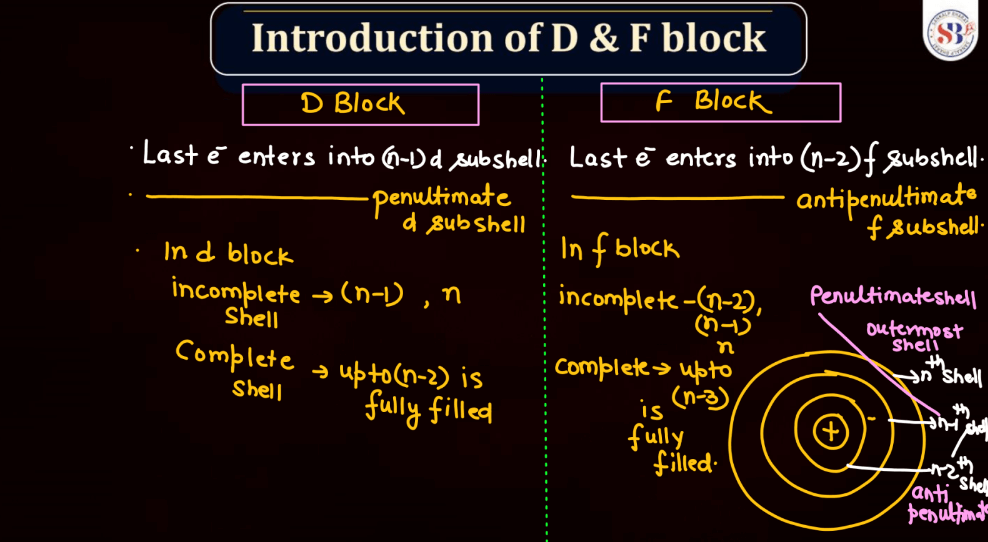
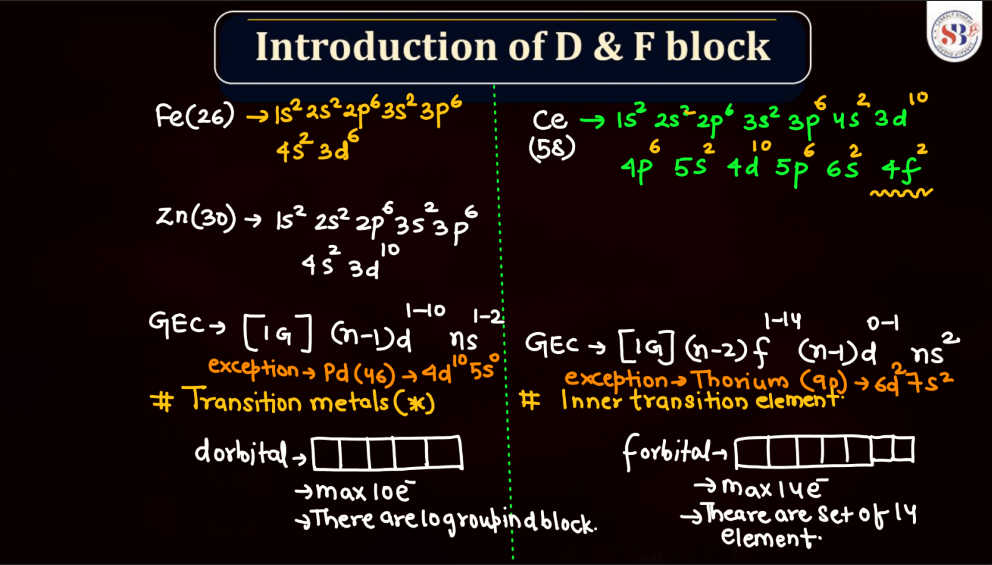
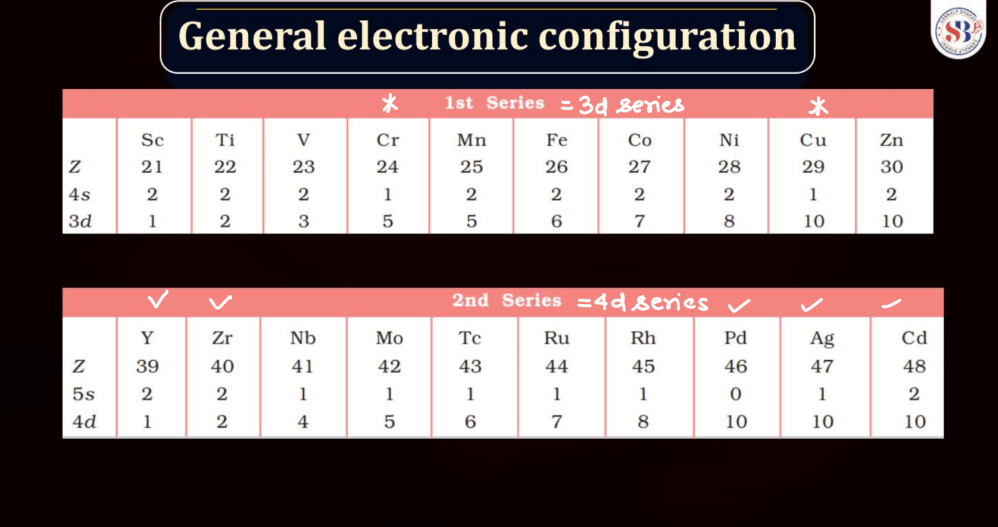
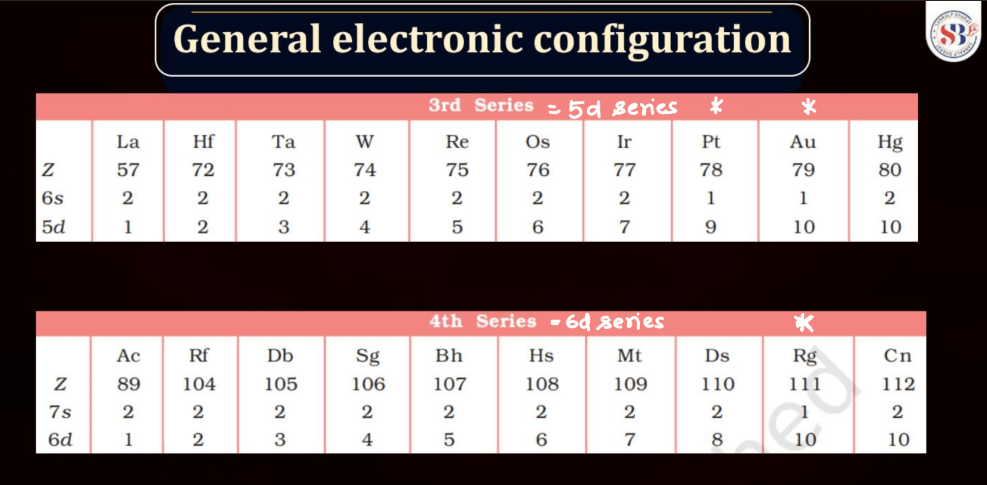
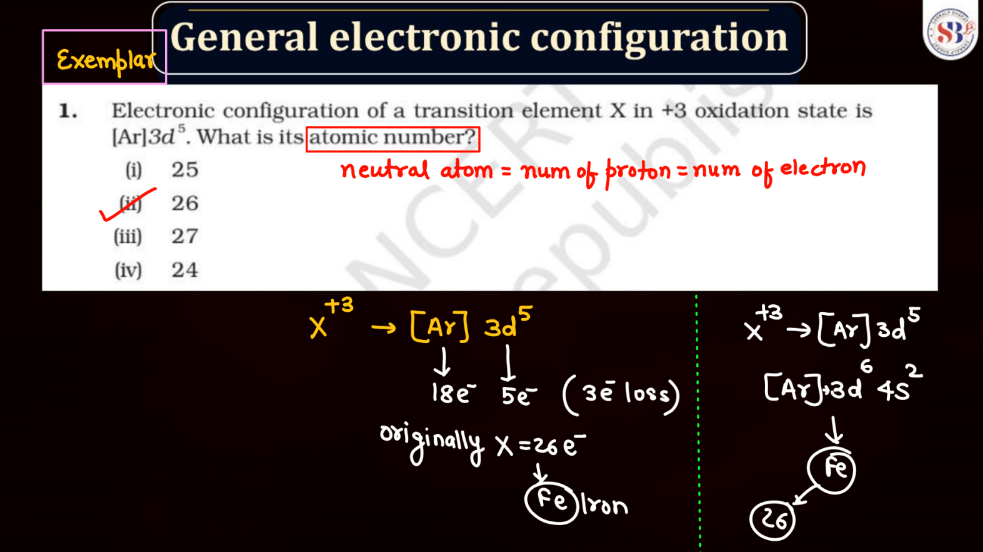
The electronic configuration of an atom describes the distribution of its electrons within various energy levels and orbitals. Electrons occupy orbitals in a specific order based on the increasing energy levels. The configuration is typically expressed using a series of numbers and letters, such as 1s² 2s² 2p 6 , where the numbers represent the principal energy level and the letters indicate the type of subshell (s, p, d, f). The Pauli Exclusion Principle dictates that no two electrons in an atom can have the same set of quantum numbers, leading to the filling of orbitals in a specific pattern.
Understanding electronic configuration is crucial in predicting an element’s chemical behavior and its placement in the periodic table. This configuration serves as a foundation for explaining the arrangement of electrons in atoms and their involvement in chemical bonding.
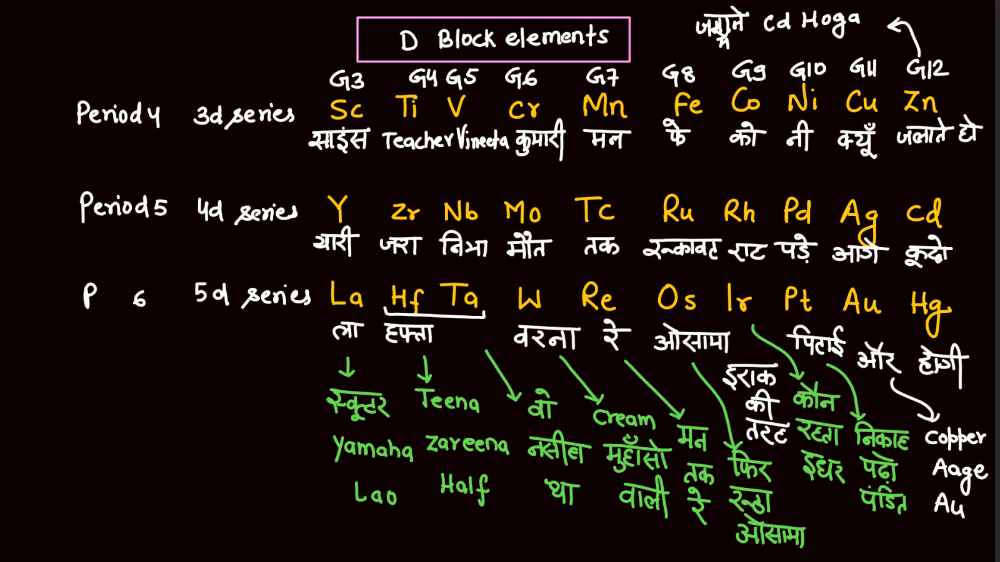
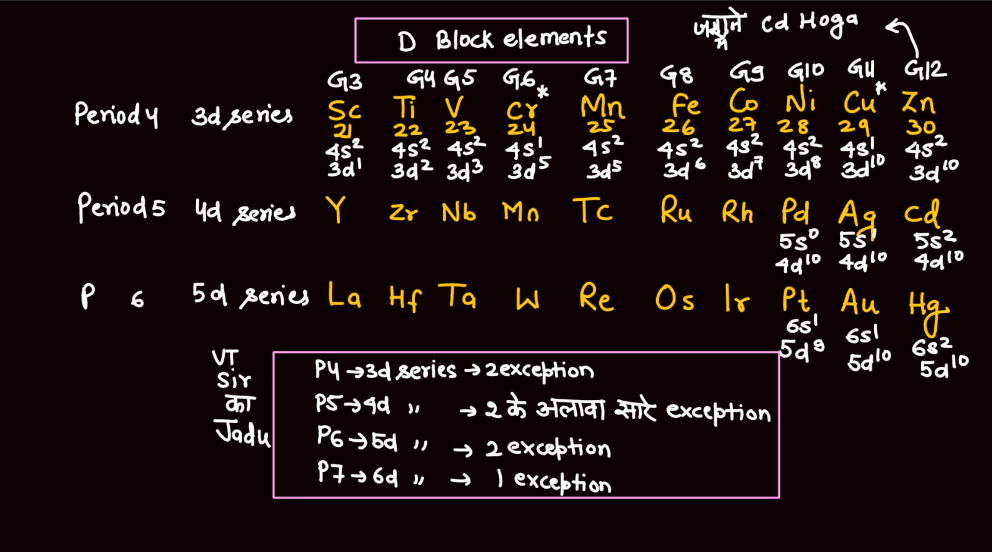
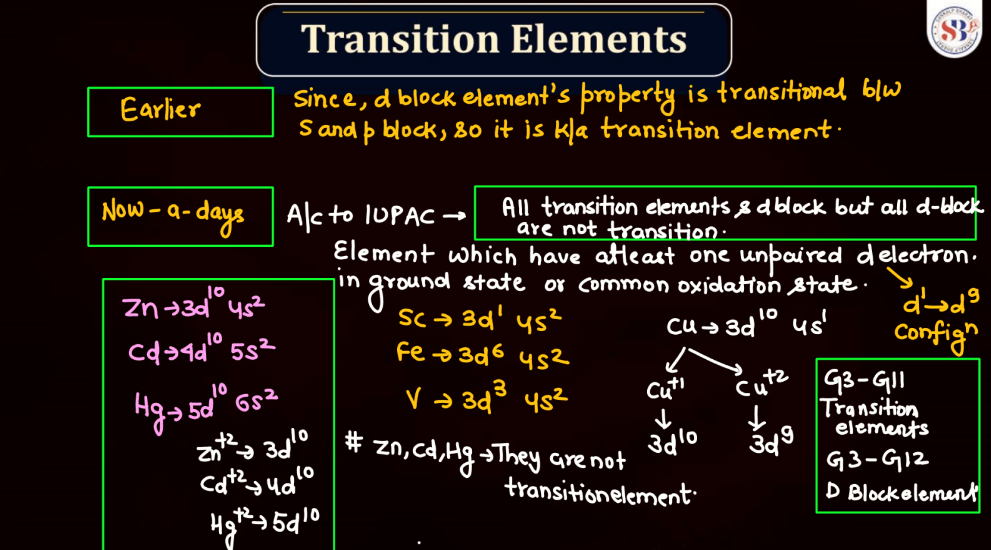
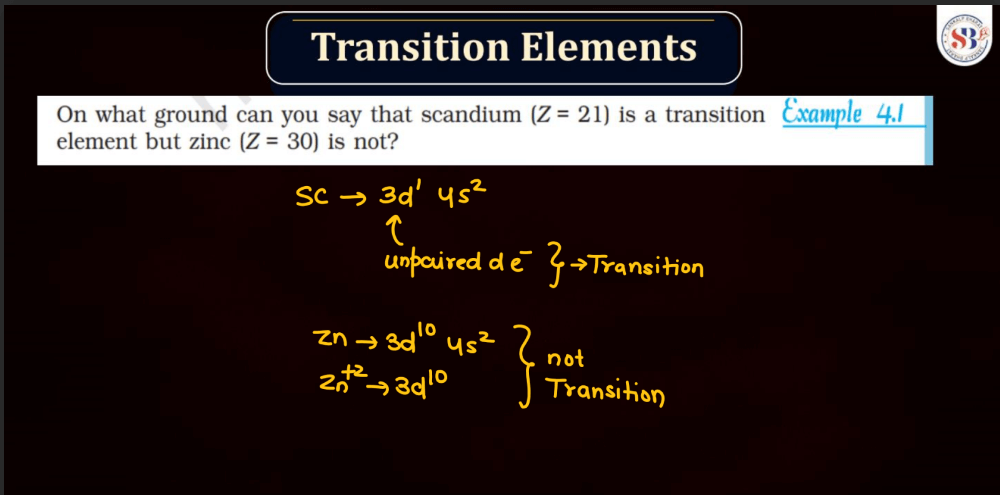
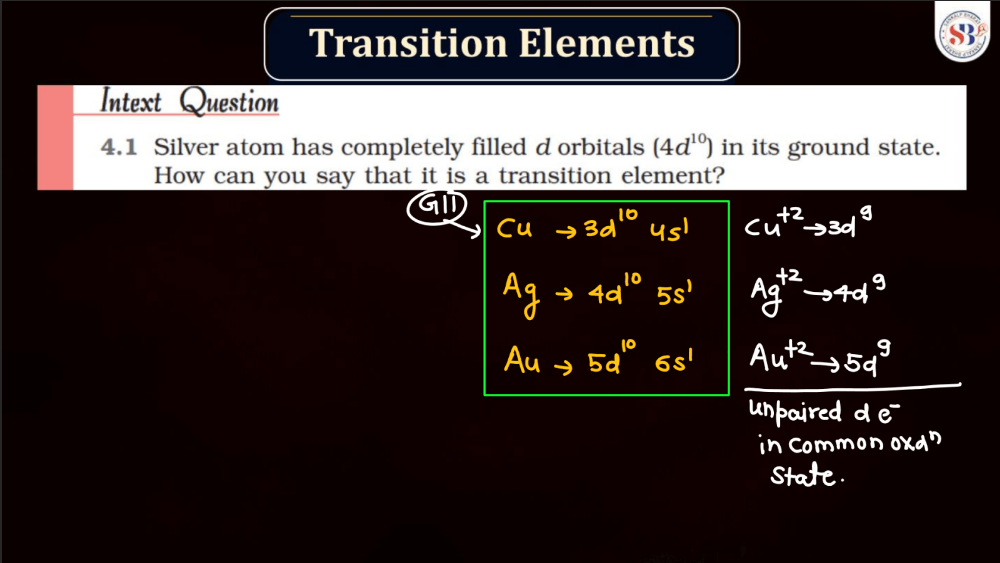
Transition elements, also known as transition metals, are a group of elements found in the middle of the periodic table. They include familiar metals like iron, copper, and zinc. What makes them unique is their electron configuration, which allows for the presence of electrons in their outer and inner energy levels. This characteristic contributes to their diverse chemical properties and ability to form colorful compounds.
Transition elements often exhibit variable oxidation states, meaning they can lose different numbers of electrons, leading to a range of chemical behaviors. Their distinctive metallic properties, such as conductivity and malleability, make them essential in various industrial applications, from construction to electronics. Overall, transition elements play a crucial role in the chemistry of materials and are integral to the functioning of many everyday items.
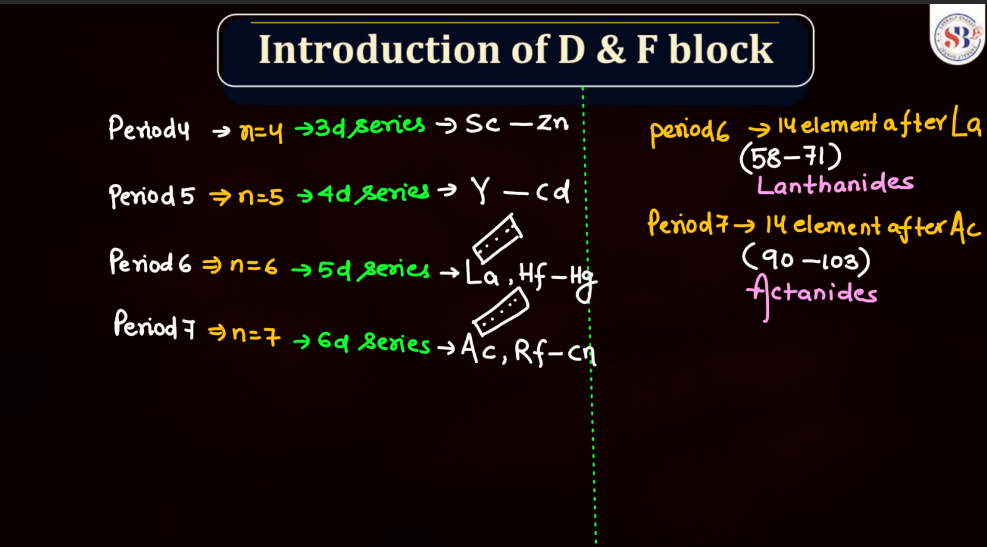
D and F block elements, also known as transition metals and inner transition metals, exhibit several common physical properties. Remember, the f-block elements are commonly referred to as lanthanides (4f series) and actinides (5f series). These elements have unique properties due to the presence of f-orbitals.
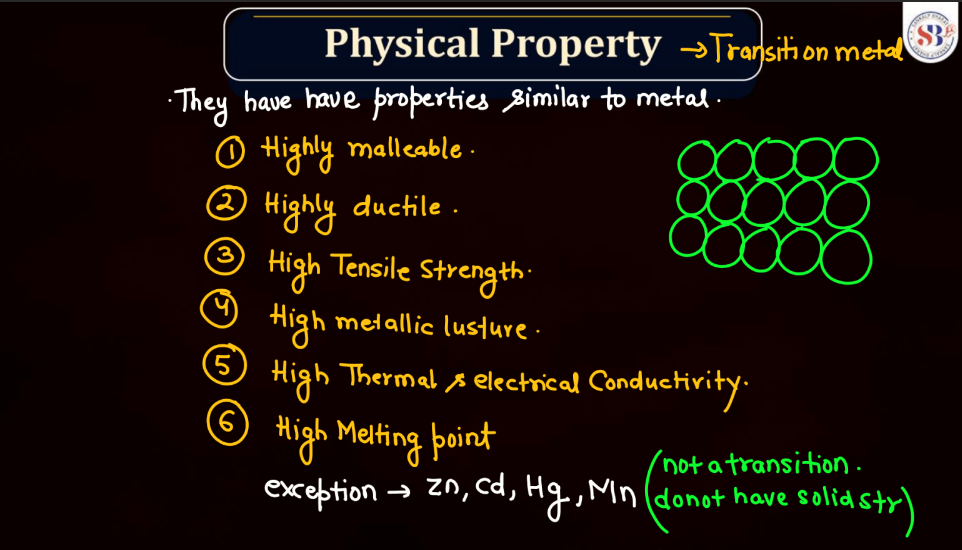
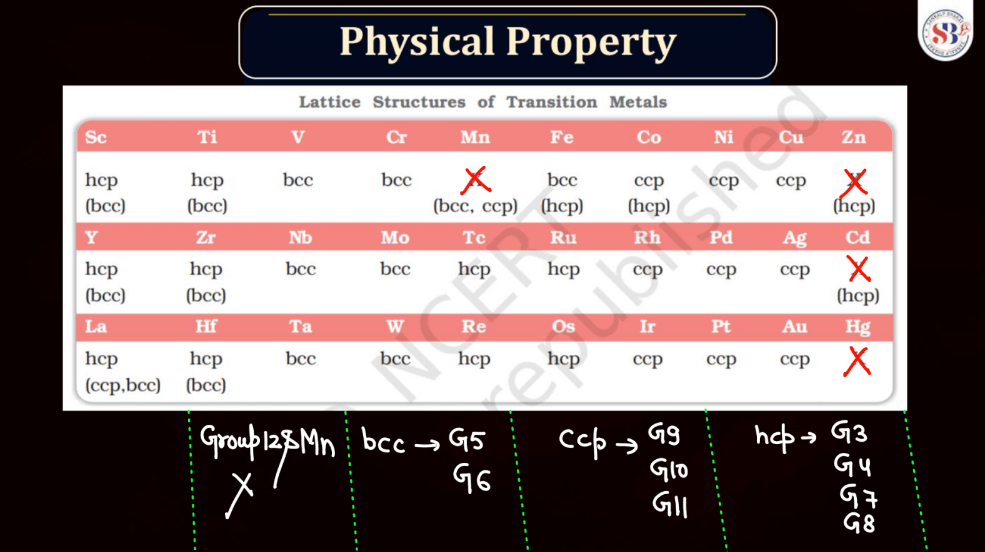
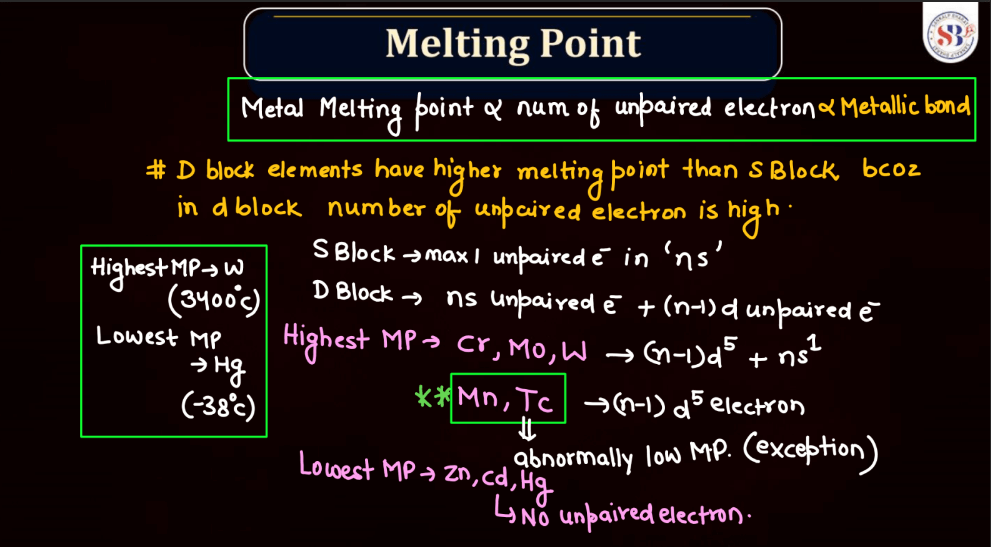
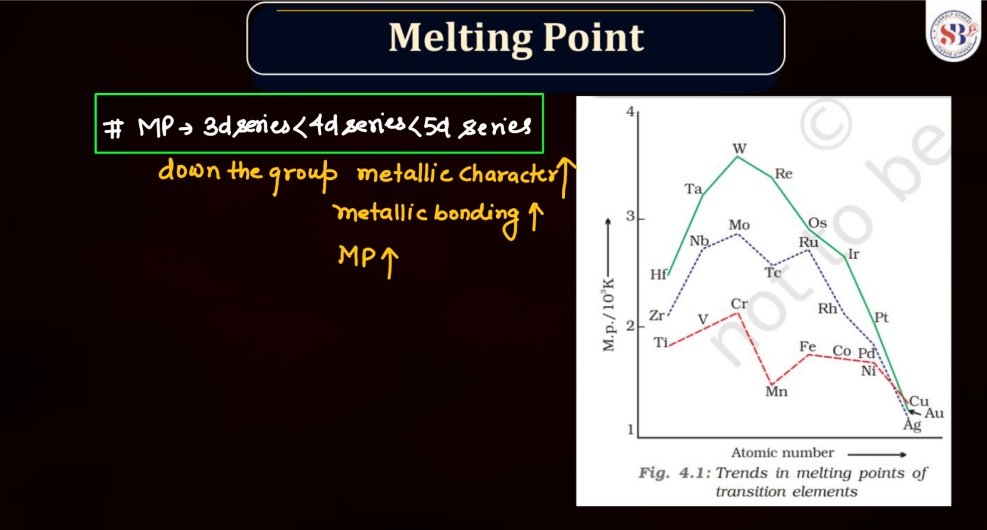
Both d and f block elements exhibit a wide range of melting points due to their varied electronic configurations and bonding characteristics. Generally, these elements have higher melting points compared to s and p-block elements. Transition metal, found in the d block, typically has high melting points owing to strong metallic bonding resulting from the delocalization of electrons. This stability arises from their ability to form multiple bonds and complex structures.
Meanwhile, f-block elements, notably the lanthanides and actinides, display diverse melting points. Lanthanides generally have high melting points, similar to transition metals, due to strong metallic bonding. Actinides, on the other hand, often have lower melting points due to the influence of their partially filled f orbitals, which can lead to weaker bonding. Overall, the melting points of d and f block elements reflect their intricate electronic configurations and bonding behaviors.
The enthalpy of atomization for d and f block elements refers to the energy required to convert one mole of these elements from a solid state into individual gaseous atoms. In simpler terms, it measures the strength of the bonds holding these atoms together in a solid. D block elements, found in the transition metals, have strong metallic bonds, making their atomization enthalpy relatively high. F block elements, often known as inner transition metals, exhibit even higher atomization enthalpy due to complex electron arrangements. The process involves breaking these strong bonds to create the energy required for this separation, which is the enthalpy of atomization.
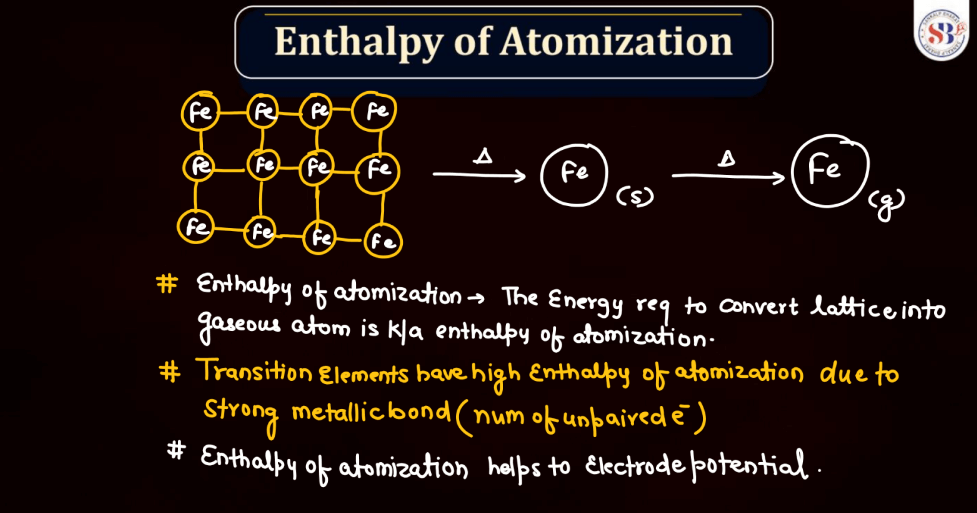
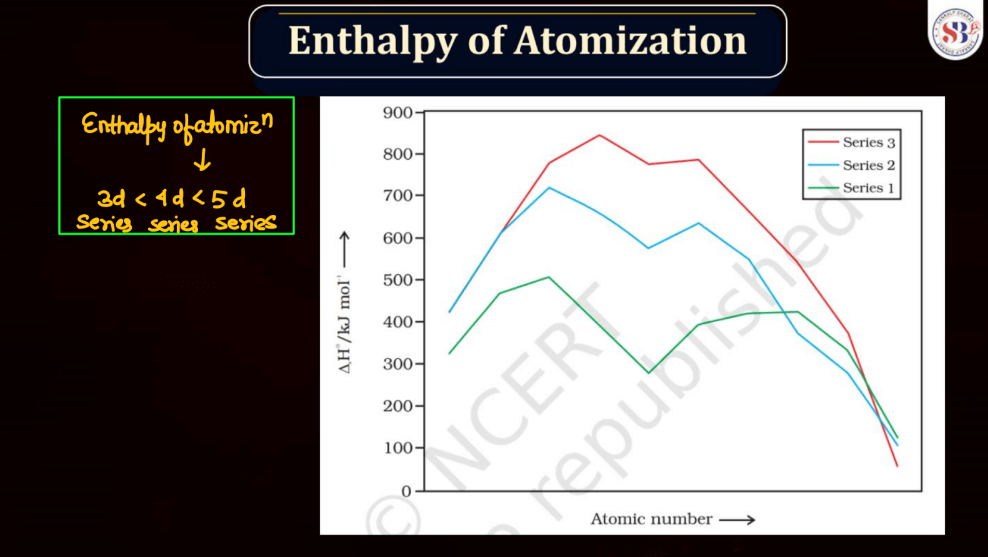


The boiling points of d and f block elements, predominantly transition and inner transition metals, vary due to their unique electronic configurations. Generally, d-block elements have higher boiling points compared to s and p-block elements. This is because transition metals have more electrons, leading to stronger metallic bonds that require more energy with a greater number of unpaired electrons or higher atomic masses tend to have higher boiling points. F block elements, specifically lanthanides, and actinides, exhibit complex electron arrangements and relatively high boiling points due to strong interatomic forces. In simpler terms, these elements resist turning into gases at lower temperatures compared to lighter elements.

The density of d and f block elements, mainly transition and inner transition metals, depends on their atomic mass and volume. D-block elements, found in the middle of the periodic table, generally have higher densities compared to s and p-block elements. This is because they are more massive and have a greater number of atoms packed into a given space. F block elements, specifically lanthanides and actinides, can exhibit even higher densities due to their relatively large atomic masses. In simple terms, the density of these elements reflects how tightly their atoms are packed together. Elements with higher density are more massive and have atoms closely packed, making them more substantial in a given volume.
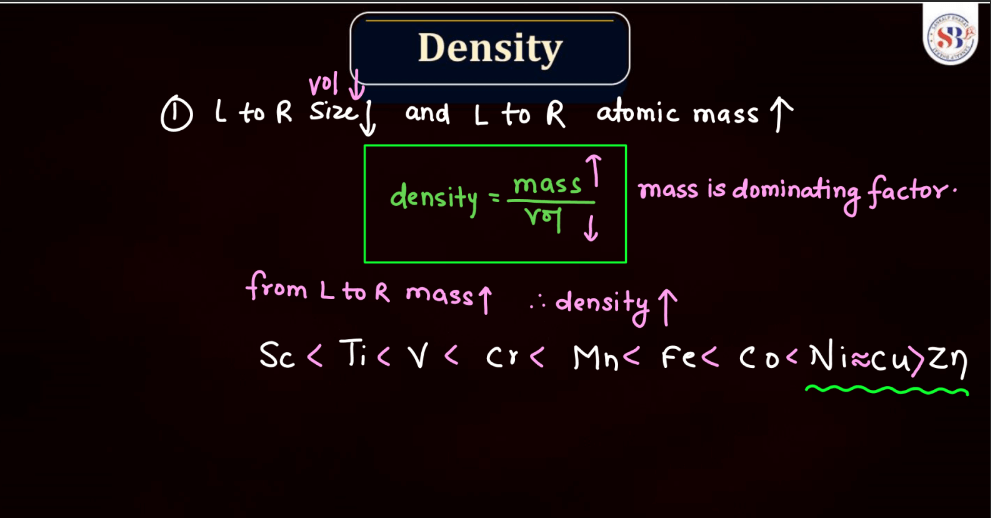
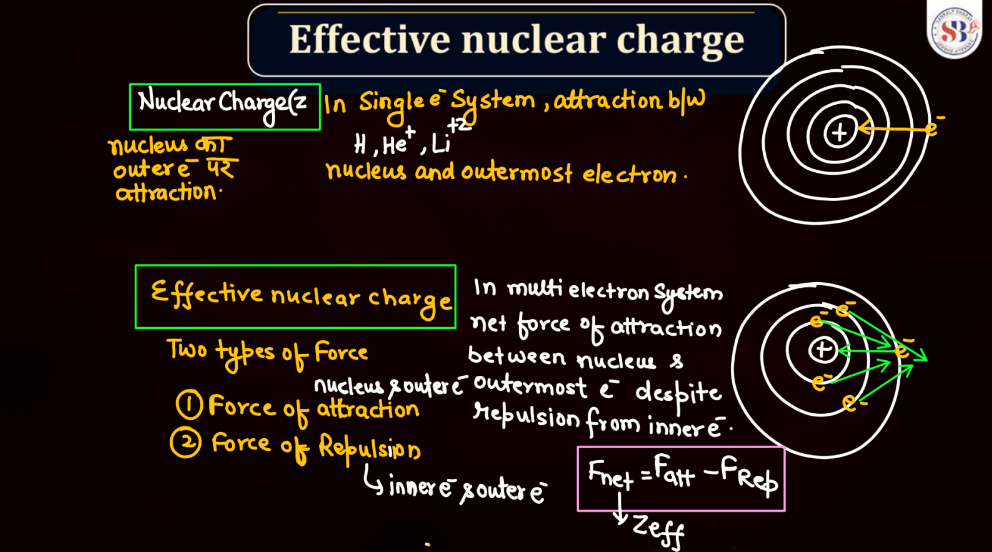
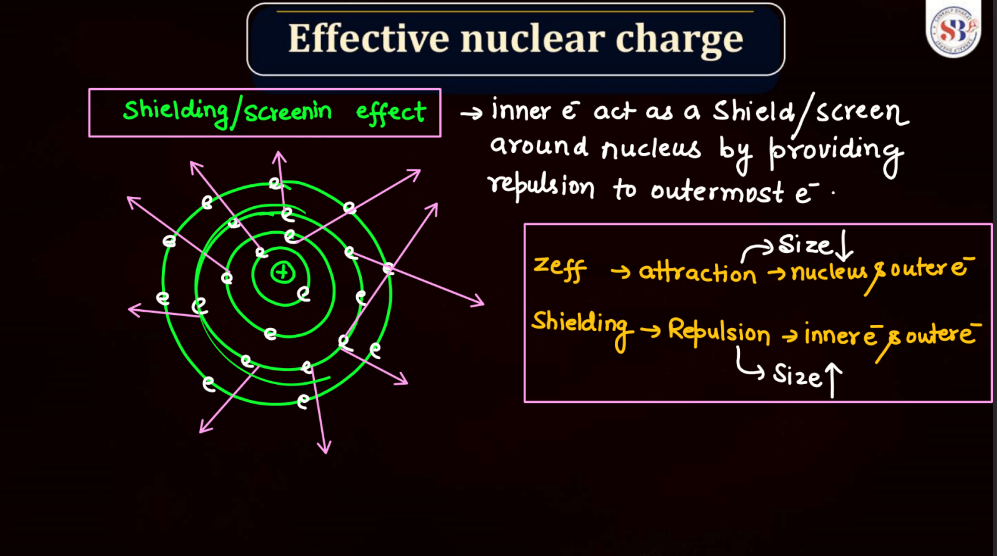
Effective nuclear charge refers to the net positive charge experienced by an electron in an atom, which is influenced by the number of protons in the nucleus and the electron’s distance from it. As electrons occupy different energy levels or shells, the inner electrons shield the outer ones from the full nuclear charge. This shielding effect results in a reduced attractive force on outer electrons. Thus, the effective nuclear charge felt by an electron is less than the actual nuclear charge. Understanding effective nuclear charge is crucial in explaining trends in atomic properties, such as ionization energy and atomic size, across the periodic table.
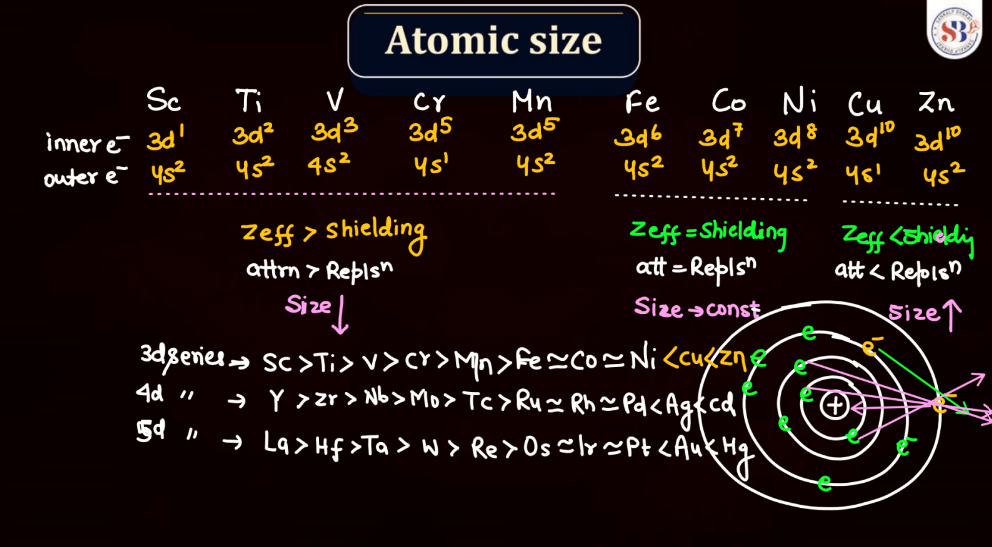
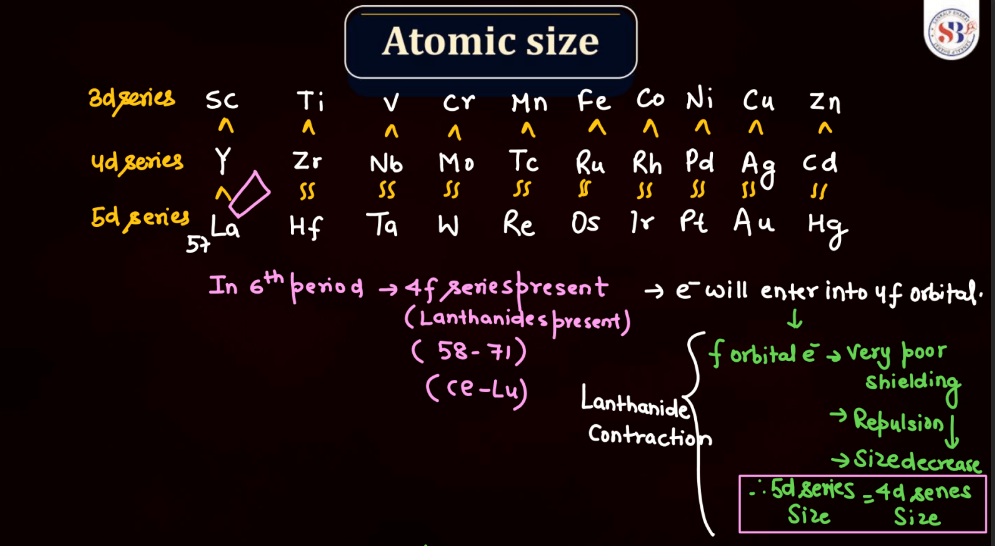
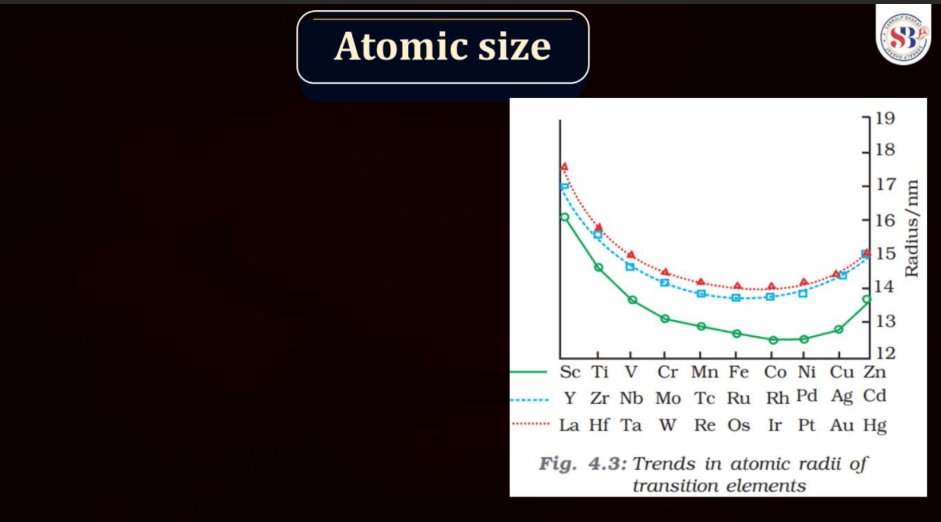
Atomic size, also known as atomic radius, refers to the size of an atom. It is typically defined as the distance from the nucleus of an atom to the outermost electron shell. Atomic size tends to increase down a group in the periodic table, as each new energy level is added, leading to a larger electron cloud. In contrast, atomic size generally decreases across a period from left to right due to the increasing effective nuclear charge, which attracts electrons more strongly. However, there are exceptions based on electron configuration. Noble gases, for example, have larger atomic sizes compared to neighboring elements in the same period due to their filled electron shells.
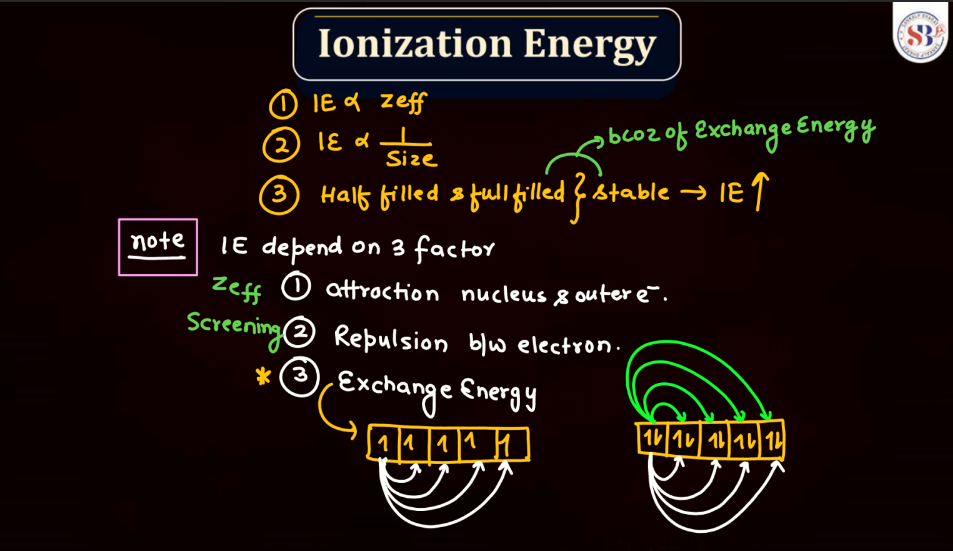
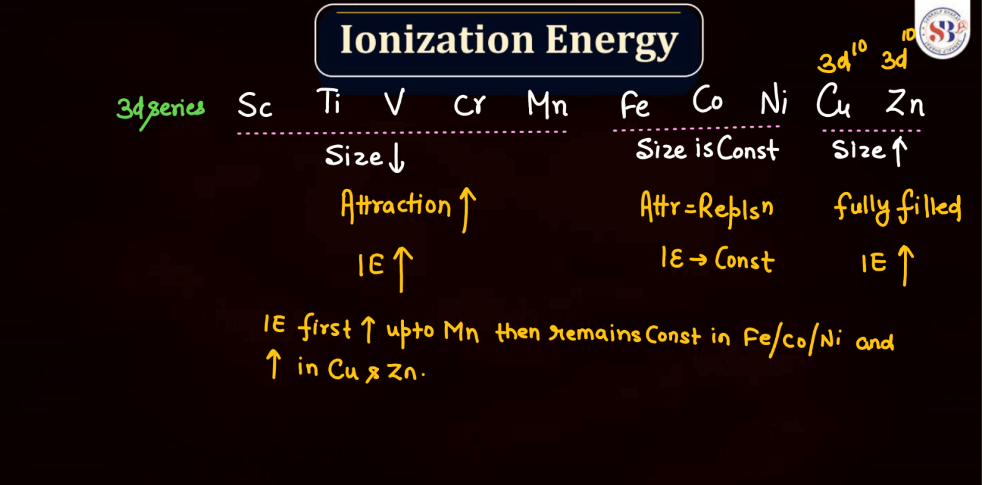
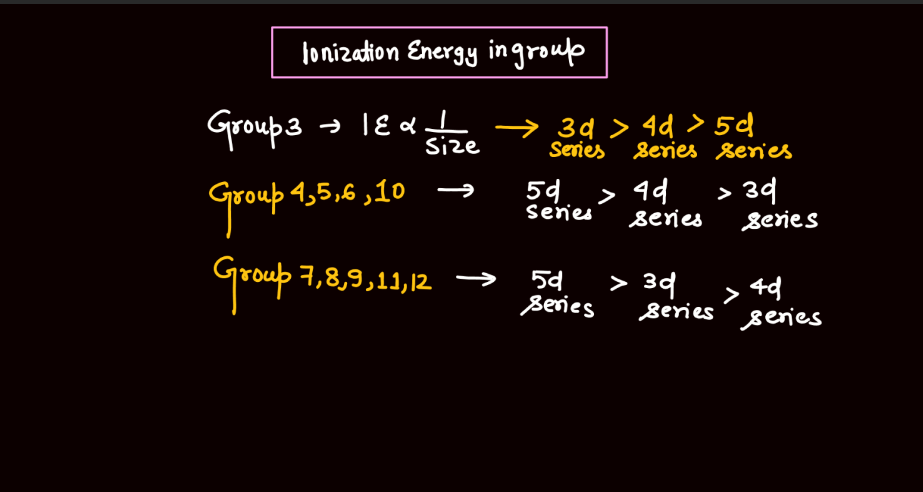
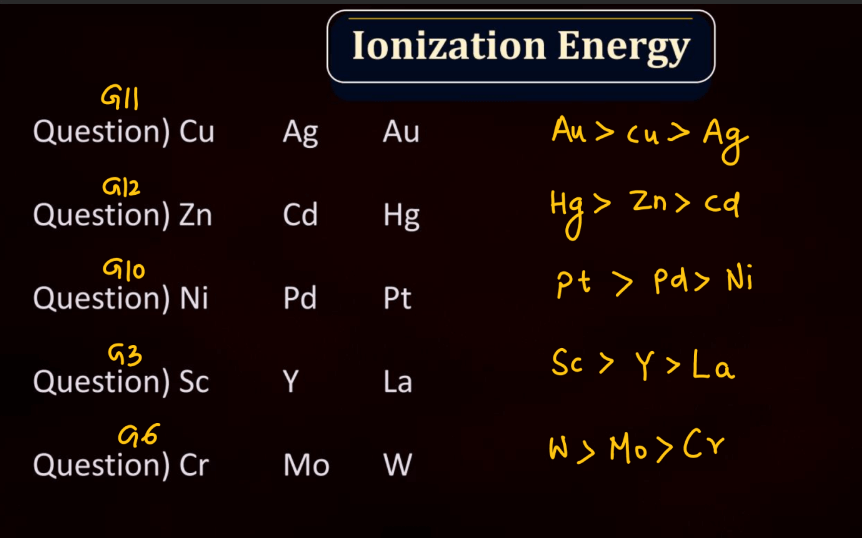
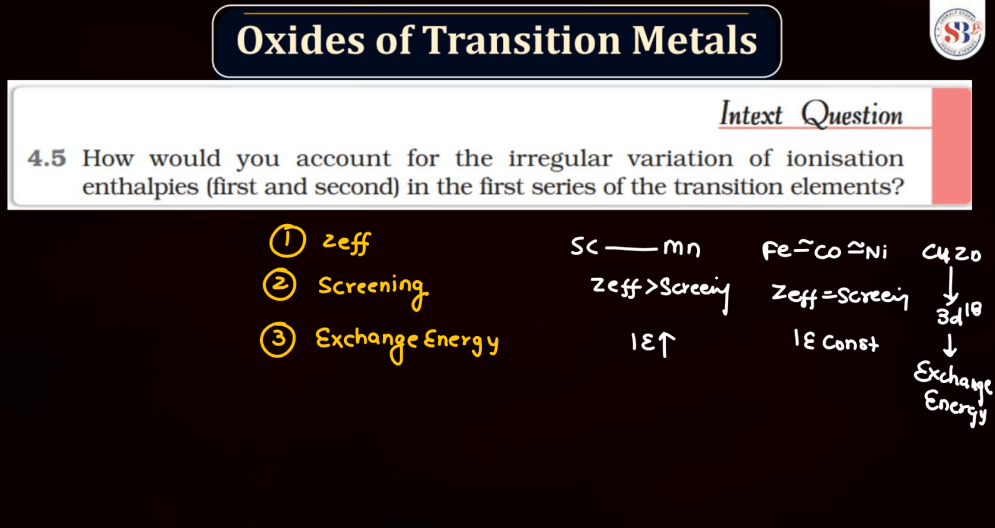
Ionization energy is the energy required to remove an electron from an atom or a positive ion. It is typically measured in kilojoules per mole (kJ/mol) or electron volts (eV). The ionization energy reflects the strength of the attraction between electrons and the nucleus. Elements with low ionization energy easily lose electrons, while those with high ionization energy resist electron removal. Ionization energy generally decreases down a group in the periodic table due to the increasing distance of outer electrons from the nucleus. Conversely, it increases across a period from left to right due to the increasing effective nuclear charge, making it more difficult charge, making it more difficult to remove electrons.
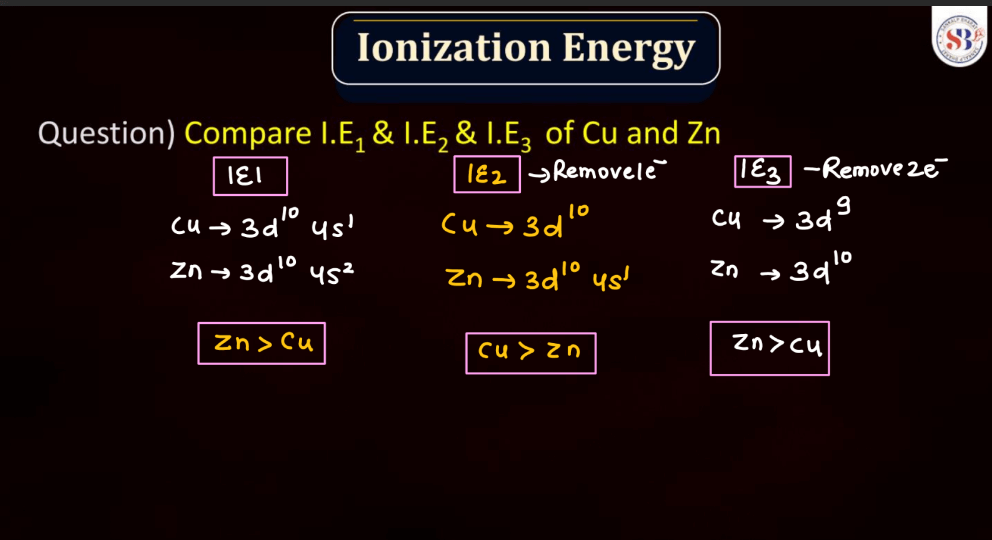
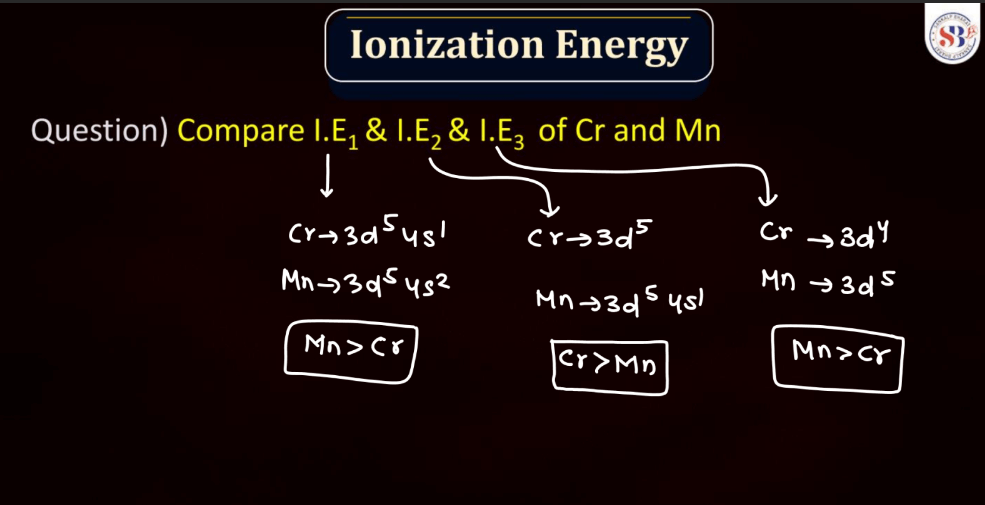


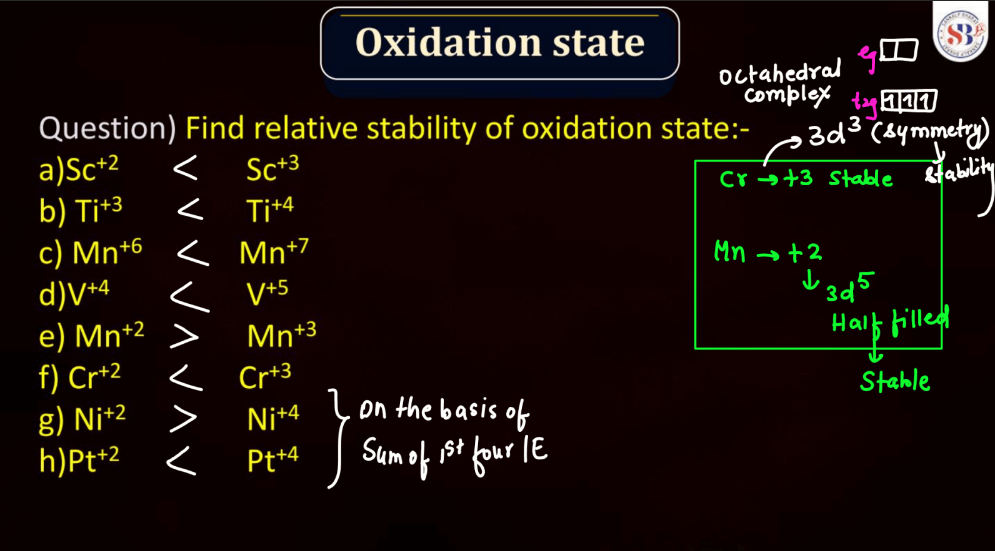
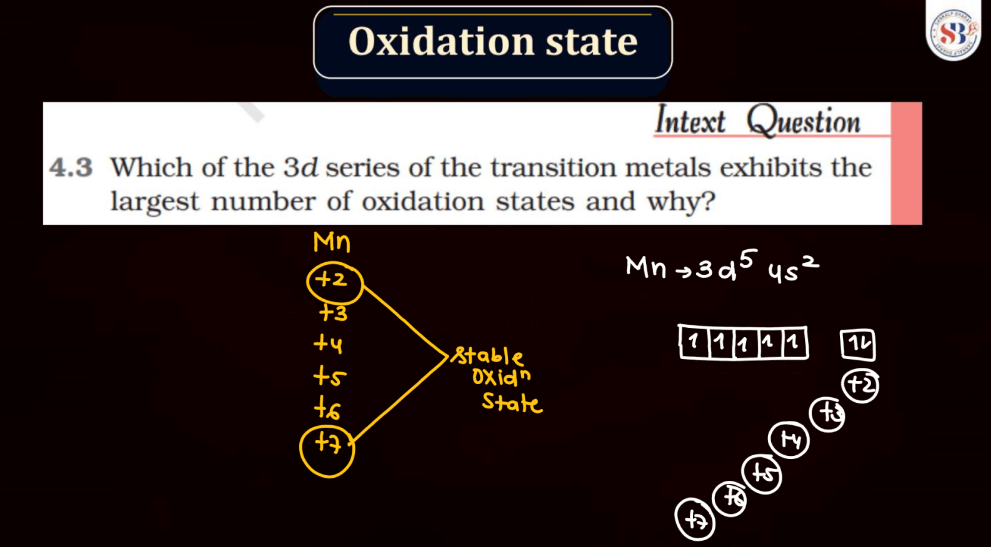
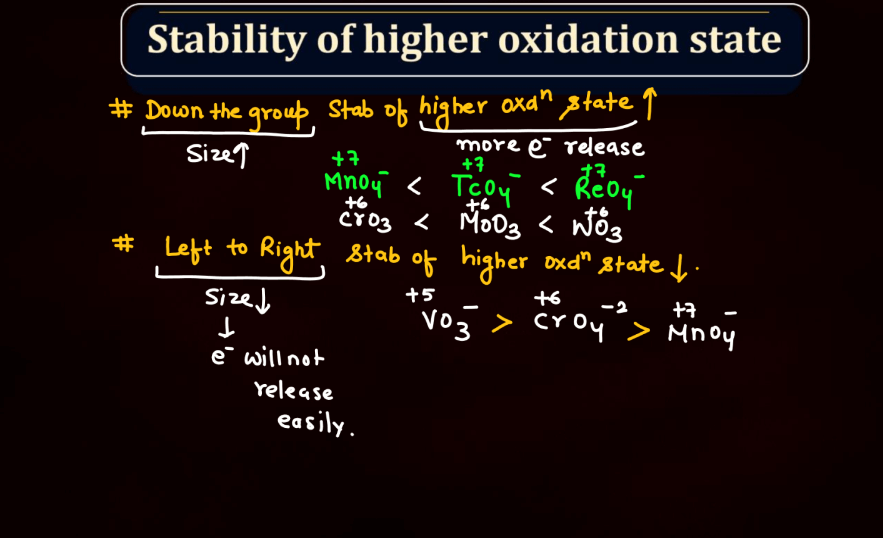
The oxidation state, also known as oxidation number, is a measure of the degree of oxidation of an atom in a chemical compound. It indicates the number of electrons that an atom has gained or lost to form the compound. The oxidation state is essential for understanding chemical reactions and electron transfer. In general, metals tend to have positive oxidation states (lose electrons), while nonmetals tend to have negative oxidation states (gain electrons). Some elements, like carbon and hydrogen, often exhibit multiple oxidation states. For example, in water (H2O), oxygen has an oxidation state of -2, while hydrogen has a state of +1. The sum of oxidation states in a compound should equal its overall charge. The concept helps balance chemical equations and predict how elements will combine in reactions.
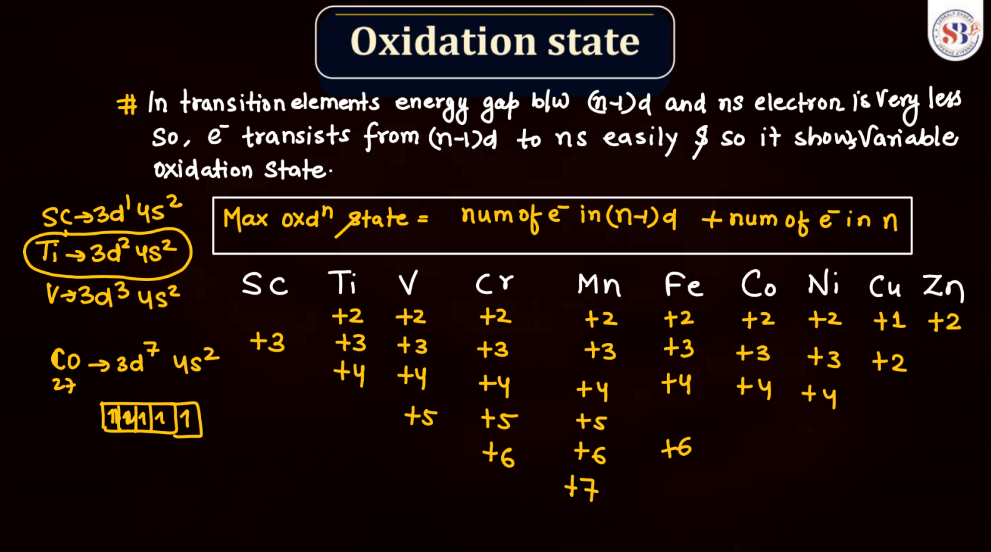
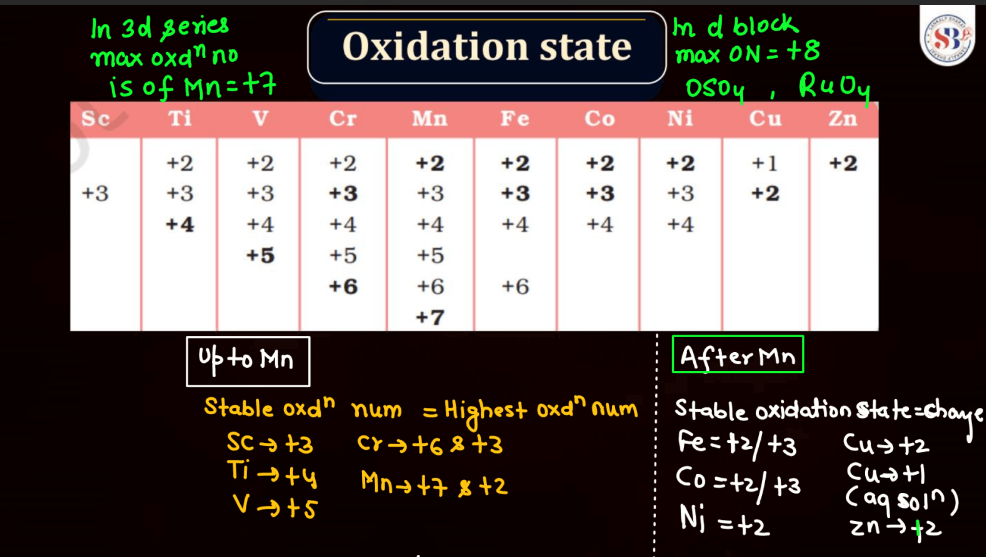

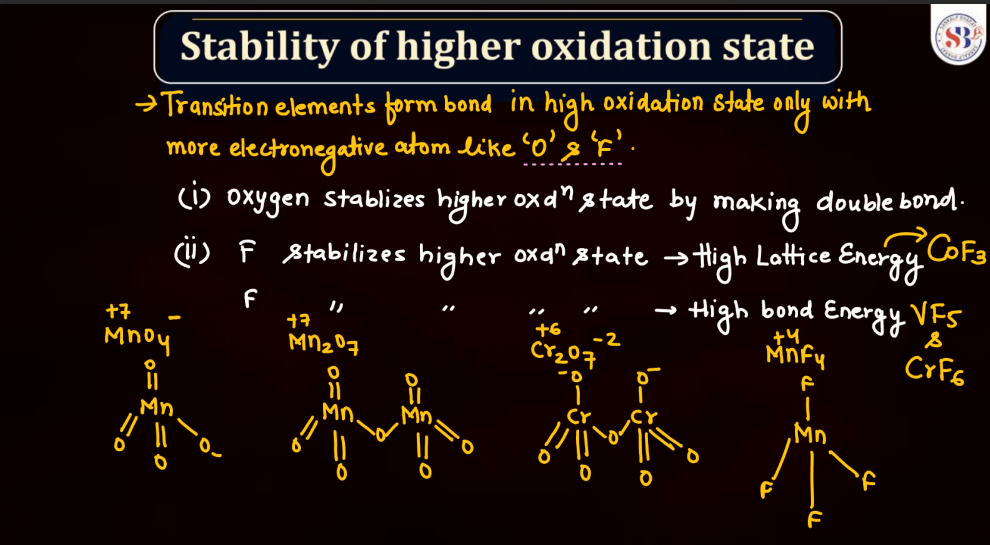
Transition metals can form a variety of oxides, exhibiting different oxidation states. These oxides often showcase diverse chemical and physical properties. For example: Copper oxides, Iron oxides, Manganese oxides, Chromium oxides, etc. These transition metal oxides can have variable oxidation states, contributing to their versatility in different chemical reactions and applications.
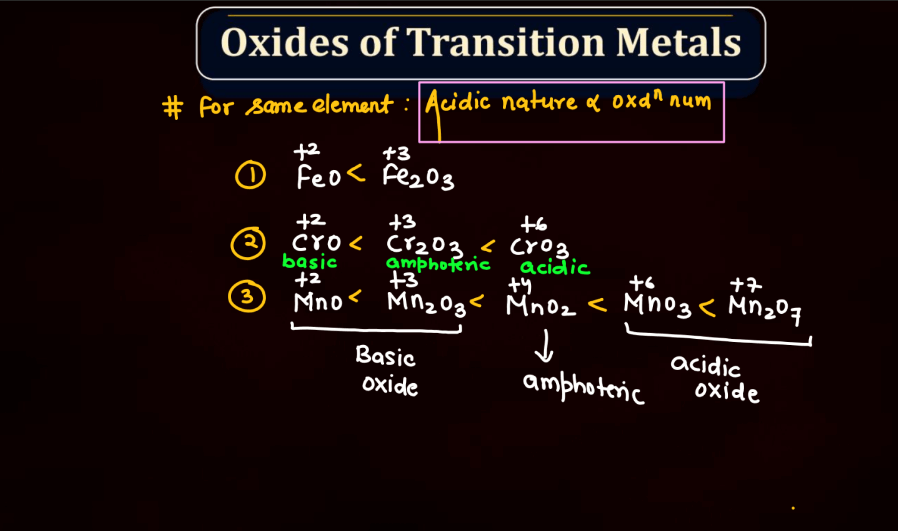
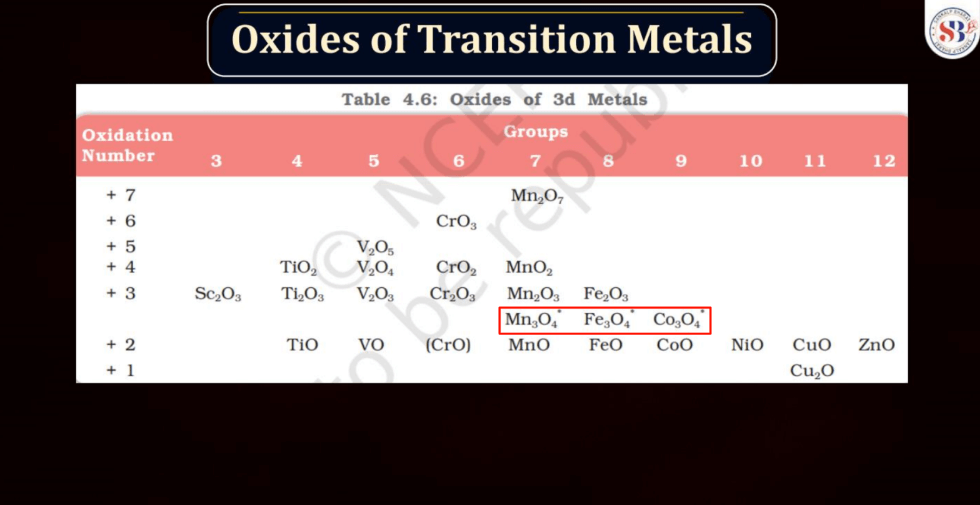
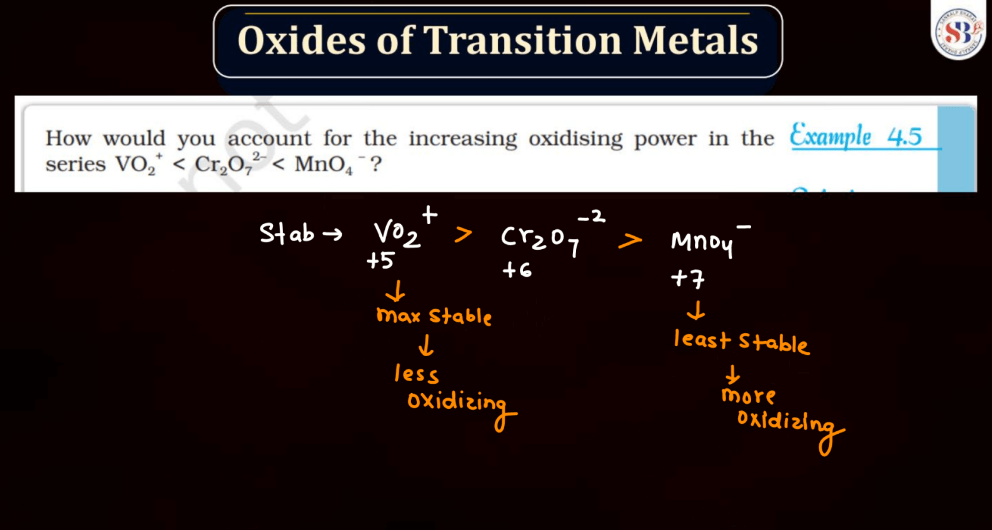
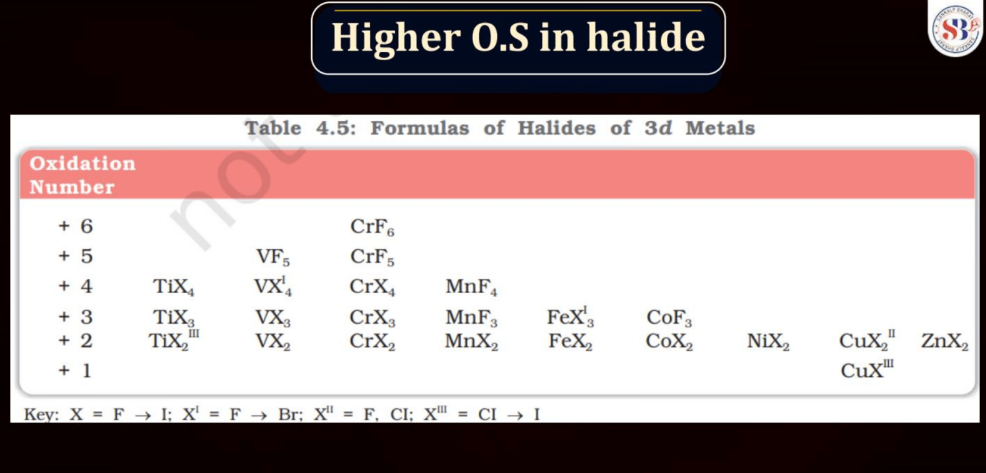
Halides are compounds formed when halogen atoms (like chlorine or iodine) combine with other elements. Normally, halogens have an oxidation state of -1. However, in higher oxidation state halides, the halogen carries a positive charge. This occurs when the halogen gains more electrons than it usually does. For instance, chlorine, which usually has an oxidation state of -1, can have a higher oxidation state in compounds like perchlorate (ClO4-). These higher oxidation state halides play important roles in various chemical reactions and are crucial in industries and environmental processes.

For a detailed study of D and F Block, Please watch the video below: